Rev’s Transcript Library
Explore our extensive collection of free transcripts from political figures and public events. Journalists, students, researchers, and the general public can explore transcripts of speeches, debates, congressional hearings, press conferences, interviews, podcasts, and more.
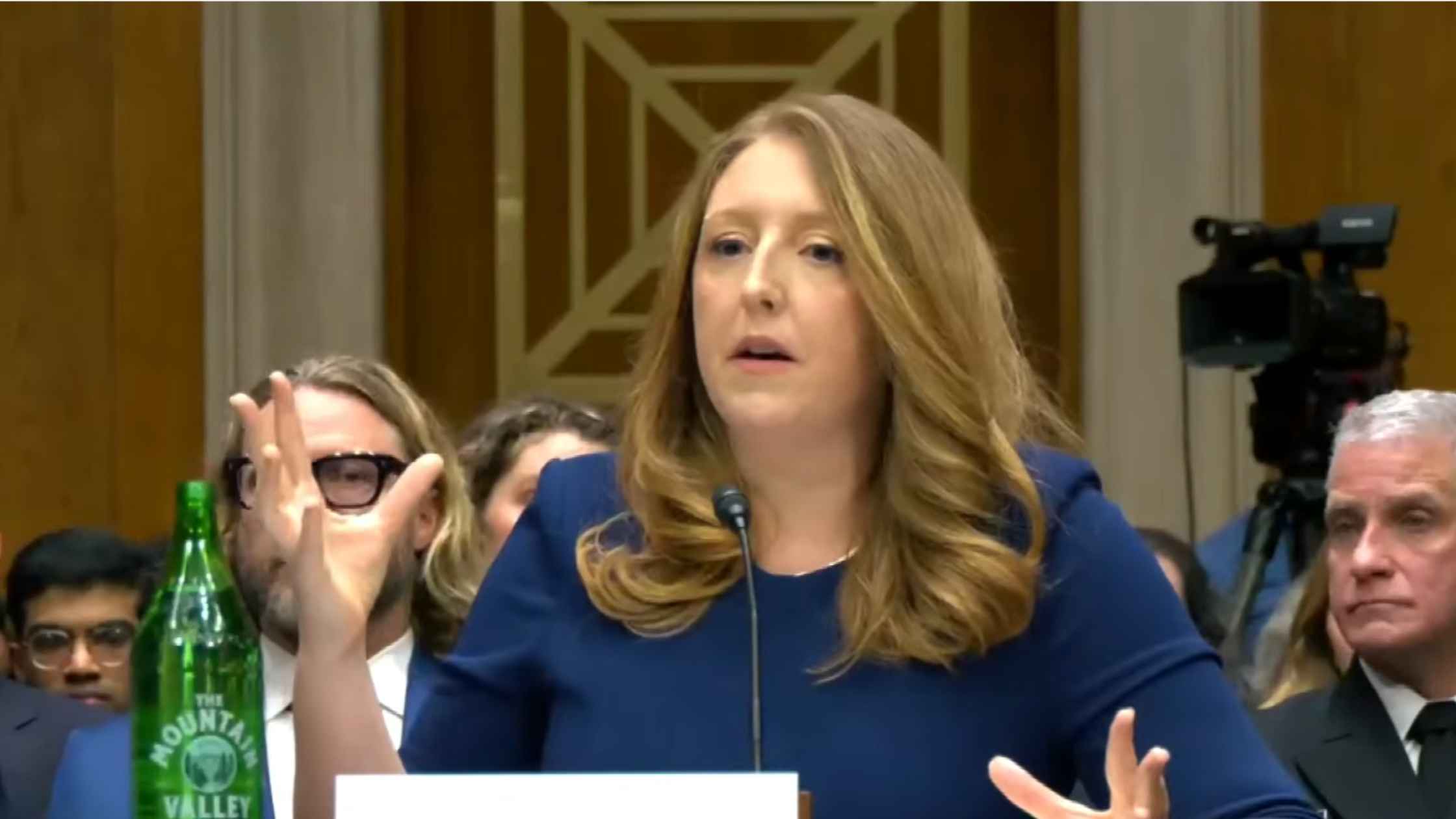
Surgeon General Confirmation Hearing
Casey Means testifies at Senate confirmation hearing for Surgeon General. Read the transcript here.

USDA Announcement
Agriculture Secretary Brooke Rollins announces initiative to update and modernize USDA facilities. Read the transcript here.

Vance and Oz on Medicare Fraud
JD Vance and Dr. Oz announce withholding of Medicaid funds in Minnesota over fraud claims. Read the transcript here.

Rubio on Cuban Speedboat Incident
Secretary of State Marco Rubio speaks after Cuba claims it carried out a deadly strike on a U.S. speedboat in Cuban waters. Read the transcript here.

United Nations Human Rights Council
The United Nations Human Rights Council starts in Geneva. Read the transcript here.

Democrat Response to the 2026 State of the Union Address
Governor Abigail Spanberger delivers the Democratic response to President Trump's State of the Union address. Read the transcript here.

2026 State of the Union Address
U.S. President Donald Trump delivers the 2026 State of the Union address. Read the transcript here.

Home Appliance Hearing
The House Rules Committee holds a hearing on the Home Appliance Protection and Affordability Act and the Homeowner Energy Freedom Act. Read the transcript here.

ICE Whistleblower Hearing
Congressional Democrats hold a public forum to hear testimony from an ICE whistleblower and others. Read the transcript here.
Subscribe to The Rev Blog
Sign up to get Rev content delivered straight to your inbox.