Rev’s Transcript Library
Explore our extensive collection of free transcripts from political figures and public events. Journalists, students, researchers, and the general public can explore transcripts of speeches, debates, congressional hearings, press conferences, interviews, podcasts, and more.

Carney Speaks after Meeting China’s Xi Jinping
Canadian Prime Minister Mark Carney speaks to the press in Beijing after meeting with Xi Jinping. Read the transcript here.

Reza Pahlavi News Conference
Exiled Crown Prince Reza Pahlavi holds a news conference on the future of Iran. Read the transcript here.

Rural Health Roundtable
Donald Trump takes part in a rural healthcare roundtable alongside RFK Jr., Dr. Oz, and other health officials. Read the transcript here.

Maria Corina Machado Press Conference
Maria Corina Machado holds a press conference after giving her Nobel Peace Prize medal to Donald Trump. Read the transcript here.

Karoline Leavitt White House Press Briefing on 1/15/26
Karoline Leavitt holds the White House Press Briefing for 1/15/26. Read the transcript here.
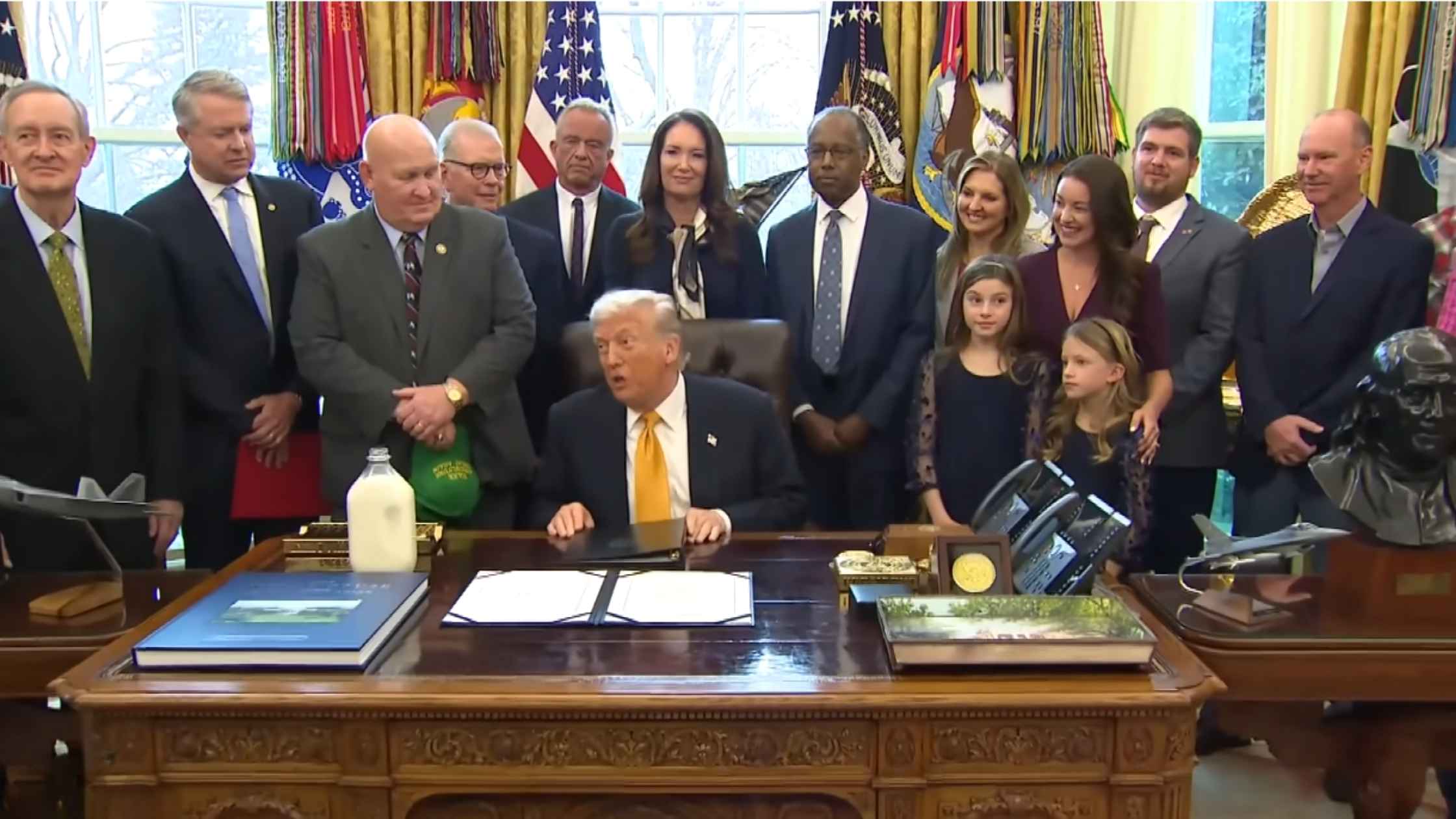
Whole Milk Executive Order
Donald Trump signs the Whole Milk for Healthy Kids Act into law. Read the transcript here.
Noem Impeachment Articles
Representative Robin Kelly says she filed articles of impeachment against Kristi Noem. Read the transcript here.

Danish and Greenland Leaders Press Conference
Danish and Greenlandic delegations hold a press conference following talks with their US counterparts. Read the transcript here.

Senate Hearing on Abortion Drugs
Senate Committee on Health, Education, Labor, and Pensions hearing on Mifepristone, one of two abortion pills approved by the FDA. Read the transcript here.
Subscribe to The Rev Blog
Sign up to get Rev content delivered straight to your inbox.